Stone : garnet-bearing amphibolite
Type : metamorphic rock derived from a magmatic rock
Age : 600 million years
Quarry : La Roche, Calanhel (Côtes d’Armor)
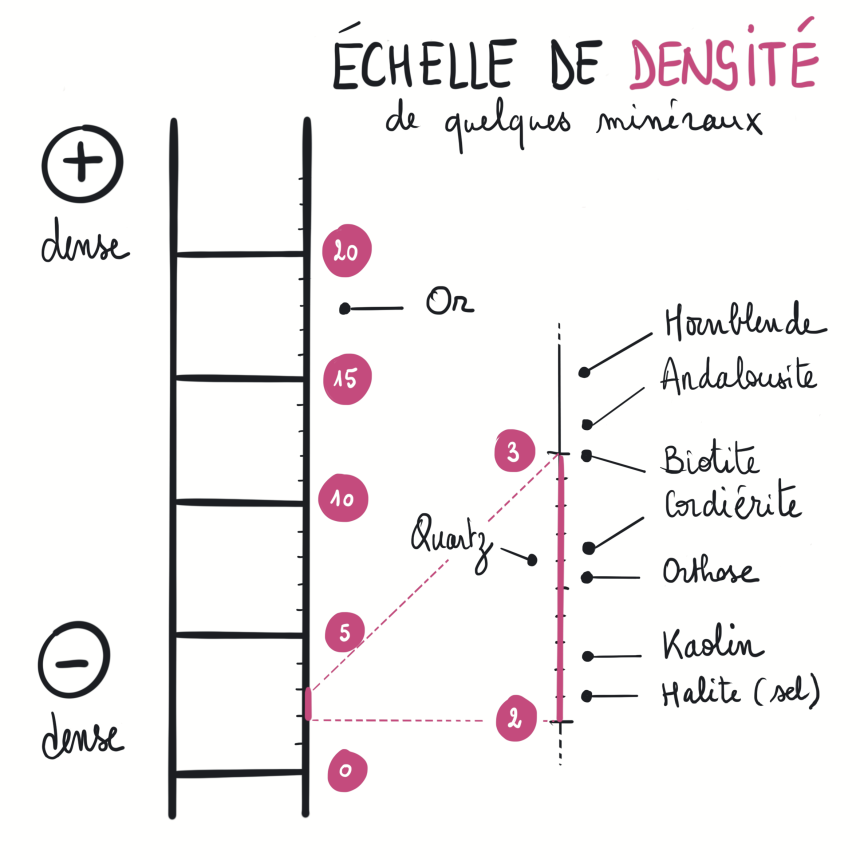
Dense and Light
The density of a rock depends on its mineral composition, but also sometimes on its structure (for example, if it has many holes, it will be lighter). Minerals are made up of chemical elements (atoms) that vary in weight. The density of a rock is therefore determined by the chemical elements it contains, as well as how those elements are arranged within the minerals. An amphibolite mainly contains amphiboles with densities ranging from 3 to 3.6.
Garnets: True Barometers and Thermometers
Garnets are relatively common minerals on Earth. But for geologists, they are absolutely exceptional. These pretty red grains are capable of recording their own history. Minerals crystallize gradually, layer by layer. By analyzing and dating the growth zones, researchers can understand the stages of garnet crystallization and thus determine how pressure and temperature conditions evolved over time. This allows geologists to trace the journey of the rock deep within the lithosphere.
Hornblende, Open Sesame: The Magic Formula
Minerals are partly defined by their chemical composition. The chemical formula of hornblende can be written as:
(Ca,Na,K)2 (Mg,Fe2+,Fe3+,Al)5 [Si6(Al,Si)2O22](OH,F)2.
This amphibole thus contains calcium (Ca), sodium (Na), potassium (K), magnesium (Mg), iron (Fe), aluminum (Al), silicon (Si), oxygen (O), hydrogen (H), and fluorine (F). Each chemical element has a specific mass.
How to Measure the Density of a Rock or Mineral?
The density (mass per unit volume) of a substance is the ratio of its mass (in kilograms) to its volume (in cubic meters). The mass of a rock can be easily obtained using a precise scale, and its volume can be determined by the amount of water it displaces when immersed in a graduated container. The density is calculated by dividing the rock’s density by the density of water at 3.98°C.